Phosphazene Base tBu‐P4 Catalyzed Methoxy–Alkoxy Exchange Reaction on (Hetero)Arenes - Shigeno - 2019 - Chemistry – A European Journal - Wiley Online Library
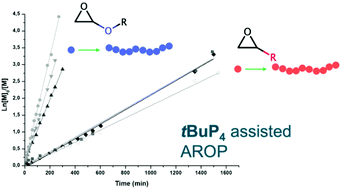
Polymerization of epoxide monomers promoted by tBuP4 phosphazene base: a comparative study of kinetic behavior - Polymer Chemistry (RSC Publishing)
Synthesis of linear and star poly(ε-caprolactone) with controlled and high molecular weights via cyclic trimeric phosphazene base catalyzed ring-openi ... - Polymer Chemistry (RSC Publishing) DOI:10.1039/C7PY01673E

Phosphazene base-catalyzed hydroamination of aminoalkenes for the construction of isoindoline scaffolds: Application to the total synthesis of aristocularine - ScienceDirect
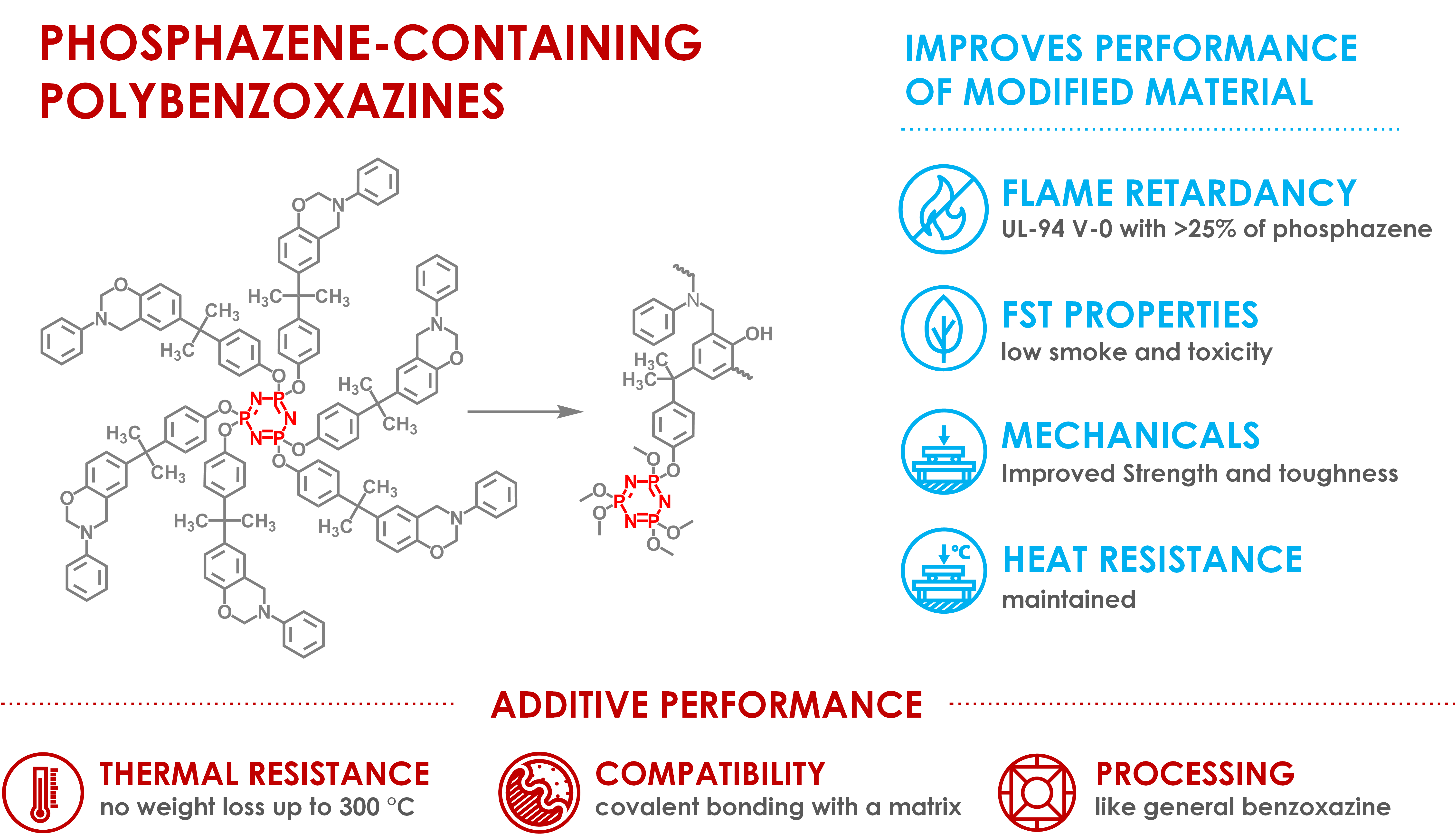
Polymers | Free Full-Text | Synthesis of Phosphazene-Containing, Bisphenol A-Based Benzoxazines and Properties of Corresponding Polybenzoxazines | HTML

Deprotonation of benzylic ethers using a hindered phosphazene base. A synthesis of benzofurans from ortho-substituted benzaldehydes. | Semantic Scholar

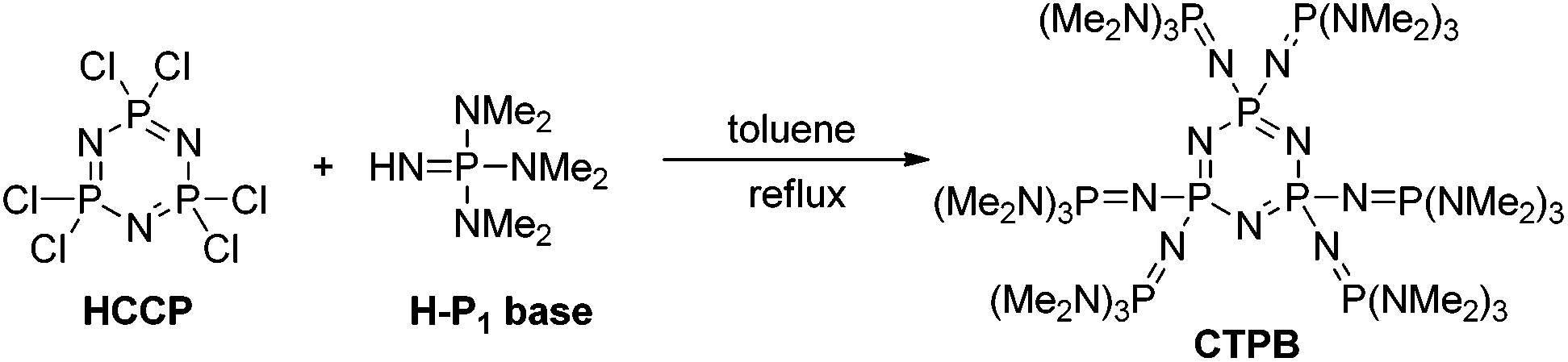
Synthesis of Tris-Phosphazene Bases with Triazine as Core and Their Applications for Efficient Ring-Opening Alternating Copolymerization of Epoxide and Anhydride: Notable Effect of Basicity and Molecular Size | ACS Macro Letters
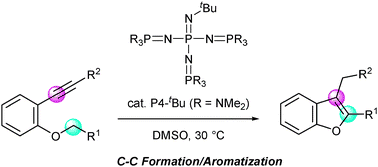
Phosphazene base-catalyzed intramolecular cyclization for efficient synthesis of benzofurans viacarbon–carbon bond formation - Chemical Communications (RSC Publishing)